Creating enzymes for sustainable chemistry

We cannot live without enzymes: they break down our food and are vital for the working of our brain and many other physiological functions. Their main job is to speed up chemical reactions within and outside cells, and make sure they take place in the right way. This makes them also useful in, for example, the chemical industry, the food industry, and for cleaning your laundry. The University of Groningen is one of the leading European centres in enzyme engineering, the fine-tuning of enzymes for practical applications.
FSE Newsroom | René Fransen
Enzymes are increasingly used in the chemical industry to make it more sustainable. Traditional chemical processes often require toxic solvents and high temperatures or pressure. By contrast, enzymes work in conditions resembling our own bodies: at a moderate temperature and in a watery environment. ‘Recent EU rules limit the use of toxic chemicals, so enzymes are a good alternative,’ says Marco Fraaije, Professor of Biochemistry at the Groningen Biomolecular Sciences and Biotechnology Institute (GBB).

What are enzymes?
Enzymes are proteins that speed up, or catalyze, chemical reactions. There are several ways in which they do this: they can bring two molecules together to react in their reaction centre, or the enzyme itself can bind to a large molecule and break it down or chemically modify it. The 3D structure of an enzyme is crucial to its function, as are the chemical properties of its building blocks, the amino acids. On top of that, enzymes often need additional molecules, called cofactors, to be active.
There are many different enzymes, and each accepts specific molecules in their reaction centre, a specificity that has developed through evolution. Their function is often that of building a new product, such as a vitamin or a neurotransmitter, or of getting rid of an unwanted product, by shredding it into smaller molecules.
At the University of Groningen, the history of identifying, studying, adapting, and even creating enzymes, dates back some sixty years. The third enzyme structure ever to be elucidated was described by UG Professor of Structural Biology Jan Drenth in 1968. ‘We are one of the leading centres in Europe in the field of enzyme engineering,’ says Fraaije. Together with his colleagues Gerard Roelfes and Gerrit Poelarends, he has taken the initiative to bring all enzyme engineering scientists at the University together in a virtual research centre. Some 100 scientists are involved, mostly based in the Groningen Biomolecular Sciences and Biotechnology Institute (GBB), the Stratingh Institute for Chemistry, and the Groningen Research Institute of Pharmacy (GRIP). Fraaije: ‘We all have different and often complementary specialities.’
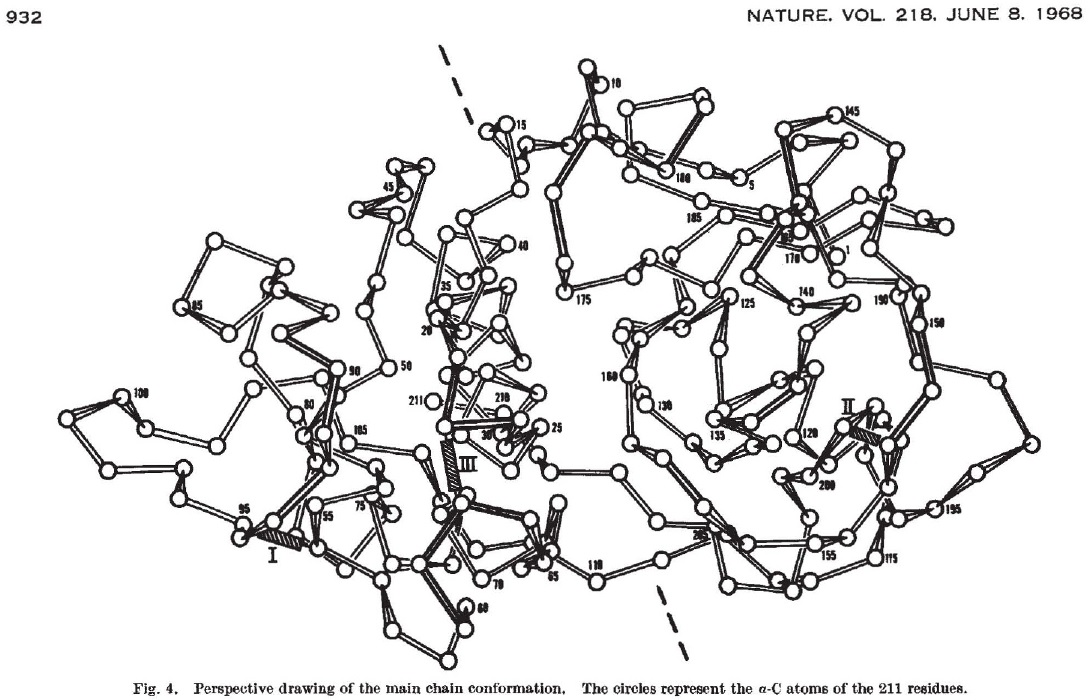
Resurrecting ancestral enzymes
For example, Fraaije has developed techniques to reconstruct the evolutionary history of an enzyme, and to ‘resurrect’ ancestral enzymes to see how they function. He recently did this for the final enzyme in the vitamin C production process. One thing he discovered is that these ancestral enzymes are often more stable. It is not entirely clear why current versions are less stable. Fraaije: ‘It may be that continued evolutionary selection for increased specificity causes a reduction in stability.’ In nature, this is not a problem, as enzymes are not meant to last in an organism, and their turnover rate is rather rapid. But to be economically viable in industrial applications, they need to last longer. Studying ancestral enzymes helps Fraaije to design more stable enzymes for industry.



Enzymes in industry and households
Many industrial processes use enzymes, for example, to break down plant polymers (cellulose) into sugars, which can then be converted into biofuels like ethanol. Food industries also use enzymes, for instance in the production of cheese or beer. And the detergents in both washing machines and dishwashers contain enzymes to break down proteins and fat or oil. More high-tech uses of enzymes include the production of specific molecules, such as building blocks for drugs. Enzymes can be used in isolation, but also inside micro-organisms: fermentation, where microbes, such as yeast, transform a product like cabbage into sauerkraut, or grapes into wine, ultimately comes down to the enzymes in the cells of these microbes.
Creating new enzymes
Gerard Roelfes (professor of Biomolecular Chemistry & Catalysis at the Stratingh Institute) has a different approach to enzyme engineering. All enzymes are catalysts, but not all catalysts are enzymes. Roelfes starts with a non-enzymatic catalyst, which speeds up the reaction he wants. Then, he couples this to a protein which has no enzymatic properties to create an enzyme that does not occur in nature. Recently, he created an enzyme containing boron in the form of a borinic acid. This type of compound is a well-known catalyst in chemistry but is not part of any biological molecules; nature does not use boron.
So why take this detour? ‘By incorporating this catalyst into a protein that is being produced in a bacterial cell, I can use directed evolution to improve its catalytic properties,’ Roelfes explains. In directed evolution, different versions of the enzyme are produced, and the one that best catalyzes the required reaction is selected and used to make a new set of variants from which the best catalyst is again selected, and so on.
Another advantage of enzymes is that they are often more efficient than other catalysts: they promote faster and more specific reactions. ‘And when used in the industry, the use of enzymes requires less energy and often produces less waste than regular catalysts.’
Directed evolution for enzyme engineering
Enzymes are proteins, and proteins are produced by cells. The information to produce a specific protein is stored in the DNA of an organism. To engineer enzymes through directed evolution, scientists have to engineer the DNA that contains the information to produce it. Thus, DNA technology is an important tool for all enzyme engineers.
Once the DNA of an enzyme has been incorporated in a cell, it is possible to make mutations that will change the enzyme at specific locations. These mutants can then be tested for the required activity. The mutant with the highest activity is used to make new mutants, which are again screened for activity. This process is called directed evolution.
However, checking all the mutants for the required activity is very time-consuming. A new way to do this is continuous evolution. Here, the product of the required reaction is linked to the survival of the cell – the more it makes, the better it will survive. So, there is no need to test all the mutants, and scientists only have to collect the survivors.

Reducing waste in the pharmaceutical industry
For Gerrit Poelarends (Professor of Pharmaceutical Biotechnology at GRIP), reducing waste is an important motivation to use enzymes for the production of drugs: ‘The pharmaceutical industry creates on average 100 kilograms of waste for one kilogram of product. That is more than in the petrochemical industry.’ By using enzymes instead of chemical catalysts to create building blocks for drugs, Poelarends wants to make production more sustainable. ‘We are also looking for renewable starting materials.’
Poelarends’ goal is to replace elements of current production processes with more sustainable building blocks. ‘There are many established production factories for drugs, and it would take a huge investment to build new factories. Our sustainable building blocks can be used in existing production lines.’ His group focusses especially on creating complex amino acids that act as neurotransmitters, which either dampen or stimulate processes in the brain. Like his colleagues, Poelarends uses directed evolution to create the most effective enzymes. In addition, he also develops enzymes that are fuelled by sunlight to achieve more sustainable transformations. In all cases, the enzymes he designs should make the production better, cleaner, and more cost-effective.
Working with industry and starting new companies
Enzyme scientists at the University of Groningen work closely with large Dutch corporations such as DSM-Firmenich. And engineering enzymes that are adopted by the chemical industry is in many cases still the ultimate aim of research in this field. Much of the research is aimed at making processes more sustainable and economically viable.
Another practical result of this research is the birth of start-up companies. Over the past two decades, a number of companies grew out of the Faculty of Science and Engineering:
CarbExplore produces functional carbohydrates for food and non-food applications. For example, they use enzymes to convert starch, sucrose, and lactose into texturizing ingredients with health benefits.
EV Biotech works on fermentation and ‘reprograms’ microorganisms to produce useful chemical compounds. This reprogramming means adapting the enzymes in the metabolic pathways of the organisms.
GECCO Biotech is an enzyme engineering company that develops sustainable enzymatic solutions for a wide range of fields, including chemistry and pharma.
These companies employ a sizeable number of scientists that were trained in the enzyme engineering groups at the University of Groningen.
More news
-
05 March 2025
Women in Science
