Evolutionary history of detoxifying enzymes reconstructed
Our body produces lots of enzymes that break down toxic substances. One class of such enzymes are the flavin-containing monooxygenases (FMOs), which are present in all tetrapods. Humans have five different FMO genes, of which the first four display the same activity. However, the fifth FMO gene triggers a different breakdown reaction. University of Groningen biochemists have succeeded in resurrecting the ancestral genes of all tetrapod FMOs to show how this divergence has occurred. Their results appear on 24 February in Nature Communications.
FMOs are present in all lifeforms, from bacteria to plants and animals. In humans, FMOs can break down a wide range of toxic substances. Of the five FMO genes present in humans, and indeed in all tetrapods (four-limbed vertebrate animals), four are responsible for the oxidation of specific groups (heteroatoms) in toxic molecules to render them harmless. However, FMO5 causes a very different chemical reaction, inserting an oxygen atom between two carbon atoms in a ketone or aldehyde compound.

Evolutionary tree
‘Our question was why this one FMO gene produces an enzyme with a different activity,’ says Laura Mascotti, who specializes in reconstructing the evolutionary history of proteins. Mascotti is a postdoc in the University of Groningen research group led by Professor of Enzyme Engineering Marco Fraaije, and the final author of the Nature Communications paper. Gene duplication is quite common, and evolutionary theory allows for different copies of the same gene to diverge. ‘We wanted to find out whether FMO5 evolved a new reaction mechanism, or whether the ancestral gene could trigger both reactions, and the other FMOs later lost one of the two.’
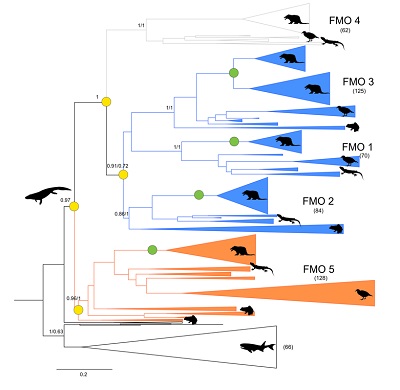
Apart from curiosity — the Fraaije group previously reconstructed the structure of four out of five ancestral FMOs from mammals — this work could help modify the enzyme’s action or design drugs that are activated by it. Mascotti: ‘For our previous paper, we compiled sequences of FMO enzymes in all living organisms, which we then used to build an evolutionary tree.’ This revealed that mammalian FMOs 1 through 4 were closely related, while FMO5 was slightly different. ‘Since all tetrapods have these five genes, we roughly knew when the ancestral gene had diverged into these two versions.’
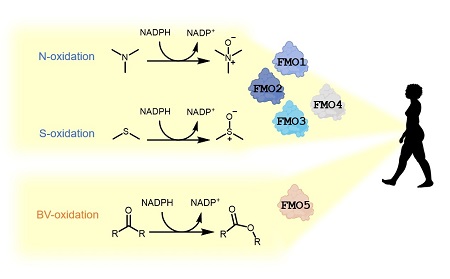
Resurrected enzymes
She calculated the most likely amino acid sequence for the common ancestral enzyme, as well as the ancestral genes for FMOs 1-4 and FMO5. ‘We did so by inferring the most likely amino acid in every position of the protein,’ explains Mascotti. ‘The result is in all likelihood not the precise original sequence, but it can still reproduce the enzyme’s activity with a high degree of probability.’ Once this work was done, the next step was to resurrect the ancestral enzymes. ‘To this end, we ordered the genes that would produce the ancestral enzymes, and expressed them in E.coli. This produced the enzymes, and we could then determine their activity.’
The resurrected enzymes produced expected and unexpected results: ‘The ancestors of the two enzyme types displayed roughly the same activity as now. However, the ancestral enzyme to all five FMOs could catalyse both reactions,’ says Mascotti. The enzyme could oxidize both heteroatoms, and ketones and aldehydes. This suggests that the present-day genes have simply lost one of these functions. Furthermore, the researchers demonstrated that changes in just three amino acids in the enzyme structure explained this difference in activity.
‘This means that the history of these enzymes is fairly straightforward,’ concludes Mascotti. ‘We believe that the ancestral gene could do everything. This gene was then copied, which meant the surplus copies could evolve more freely, resulting in different enzymes for the two functions.’ Interestingly, this happened at a time when tetrapods moved from the ocean to the land. ‘Plants produce a lot of toxic metabolites, so there was a selective advantage for animals that could break down these toxins efficiently.’
Drugs
The researchers also discovered that the cofactor which the enzymes use is important for the type of reaction they trigger. ‘Amino acids that are not part of the active site but that interact with the cofactor appeared to be vital. Usually, cofactors are just electron donors, but in this case they determine the type of activity, which is quite unique.’ This knowledge about the relation between enzyme structure and function is important to manipulate detoxifying enzymes, or to design inhibitors that would reduce breakdown of drugs.
Mascotti: ‘We have satisfied our curiosity about the history of these enzymes, and uncovered new insights into the way they function. Furthermore, we could only do so by collaborating in a very diverse team, with specialists in enzymology, evolution, and protein structure.’
Reference: Gautier Bailleul, Guang Yang, Callum R. Nicoll, Andrea Mattevi, Marco W. Fraaije & Maria Laura Mascotti: Evolution of enzyme functionality in the flavin-containing monooxygenases. Nature Communications, 24 February 2023
More news
-
26 January 2026
Science for Society | The AI chip of the future
