Uroplakin Complex
Structure of the luminal plasma membrane protein (uroplakin) complex from urinary bladder
The apical surface of mammalian urinary bladder epithelial cells is covered by numerous protein plaques. These plaques (also named Asymmetric Unit Membrane; AUM) are believed to form a diffusion barrier and play a role in strengthening and stabilizing the apical surface through interactions with the underlying cytoskeleton. The plaques contain four integral membrane proteins, called uroplakin Ia, Ib, II and III. They form complexes arranged in a well-ordered hexagonal array. AUM provides a good model system to study how several mammalian integral membrane proteins interact to form a highly organized supramolecular assembly which is able to accommodate the dynamic environment related to the strongly varying surface area of the epithelium. The complex represents one of the few examples of a mammalian membrane whose protein subunits form a 2-dimensional (2D) crystal in situ, making it directly accessible to structure determination by electron-crystallographic methods.
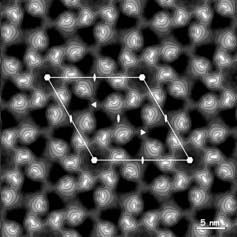
Electron micrographs are recorded on a Gatan slow-scan CCD camera attached to a Philips CM200FEG cryo-electron microscope, using a semi-automated specimen selection and data recording system. Specimens were tilted up to 55°. Analysis of the electron micrographs shows that native crystalline patches with a diameter of ~0.5 µm exist, with p6 symmetry extending to a resolution of 1.2 nm or better. A projection map calculated from an untilted crystal is shown in the figure left. Each hexamer comprises a ring of six inner and six outer domains at a radius of 5.7 and 9.2 nm respectively.
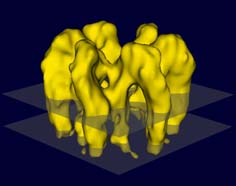
Data from images of tilted and untilted crystals were merged to reconstruct the 3-D density distribution. Fig. 2 presents an iso-surface model of the hexameric complex. The mass is distributed strongly asymmetrically with respect to the membrane (indicated by the two transparent planes), with most of the mass protruding from the luminal face. The total thickness of the complex is appr. 13.5 nm. Both domains in the asymmetric unit traverse the membrane and protrude about 2 nm from the membrane on the cytoplasmic side. The two domains are bridged on the luminal side forming a stretched arc.
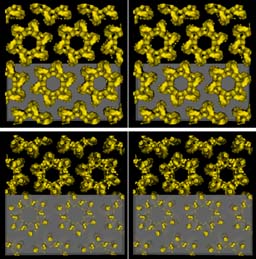
In the plasma membrane the hexameric complexes are packed in a hexagonal lattice. The stereo picture on the left shows the packing from the luminal (top) and from the cytoplasmic side.
The location of the four different uroplakins forming the complex and the different putative trans-membrane helices are not resolved at this stage. Labeling using specific antibodies or gold markers and improving the resolution beyond 1 nm should solve these questions.
