Hydrogen seeps into nooks and crannies

Could hydrogen replace natural gas in the future? Aside from the question of whether this would always be the best choice for specific applications, the infrastructure that we are now using to store and transport natural gas is not readily usable for hydrogen. Because hydrogen is a much smaller molecule than natural gas, it can easily leak. Even worse, despite its small size, hydrogen can affect larger materials and make them as brittle as glass.
FSE Science Newsroom | Text Charlotte Vlek | Images Leoni von Ristok
Suppose we have managed to produce green hydrogen, with high efficiency and for various applications. If we want to use hydrogen as a ‘green battery’ for storing variable wind and solar power, however, we must be able to store hydrogen for an extended period of time.
In the ground
For natural gas, temporary storage is already quite common: a surplus in summer is stored for winter, sometimes in existing gas fields or salt caverns underground. ‘For example, this is already being done in Zuidwending, in the province of Groningen,’ explains geologist Johannes Miocic, who is investigating whether something similar is possible for hydrogen. He quickly adds, ‘But that will not be stored in the Groningen gas field! That’s much too sensitive politically, given the earthquakes that have occurred here as a result of gas extraction.’



Studying rock samples
Subsurface natural gas is typically located in tiny spaces in between sand grains, within the larger rock layer (sandstone) deep underground. ‘It’s not a large empty space, as some people imagine,’ explains Johannes Miocic. This rock layer is present underneath the entire area of the Netherlands, including below the sea. It is covered by caprock (mudstone), an impermeable layer of several tens or hundreds of metres thick, which keeps the gas from moving upwards.
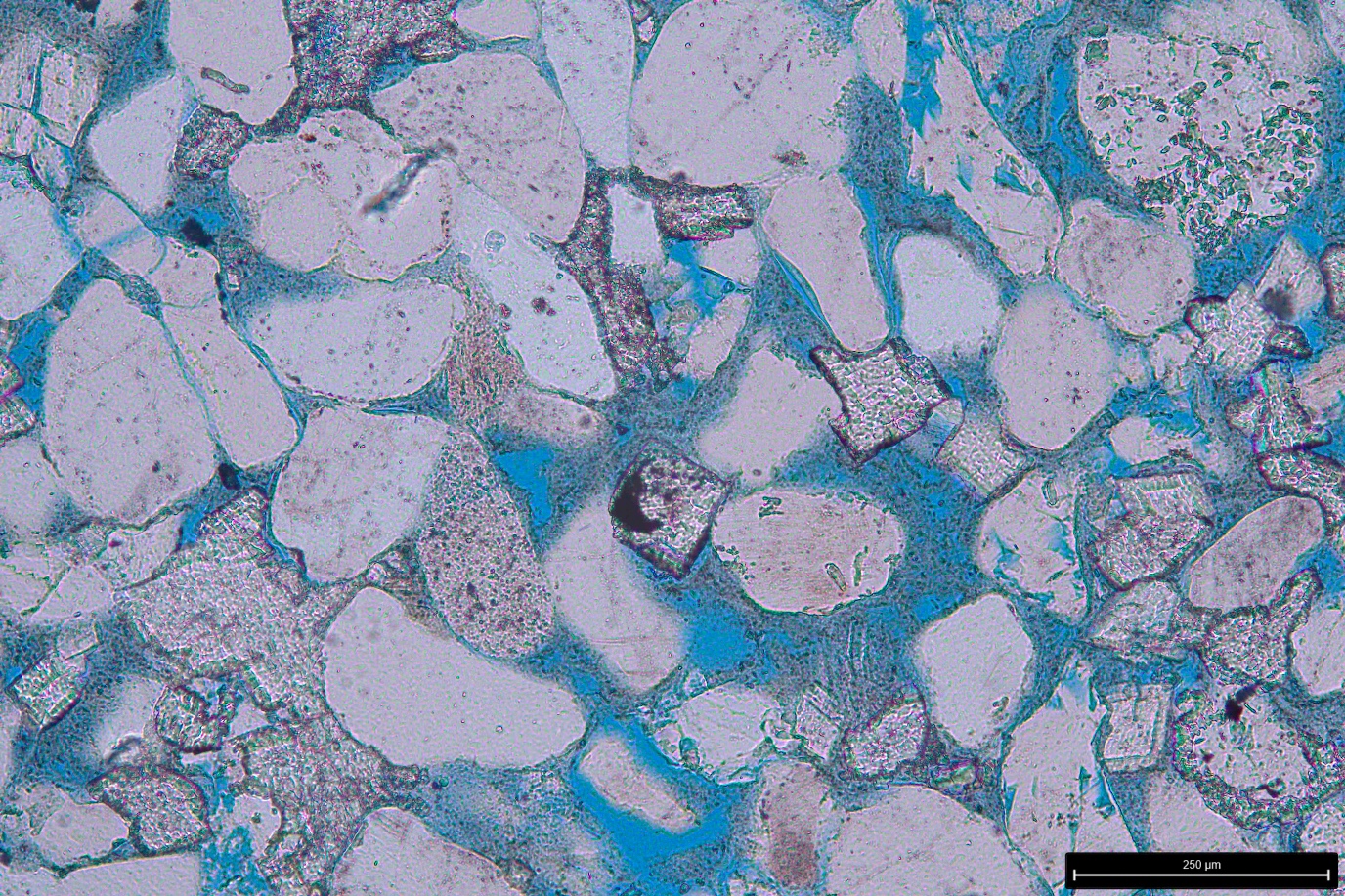
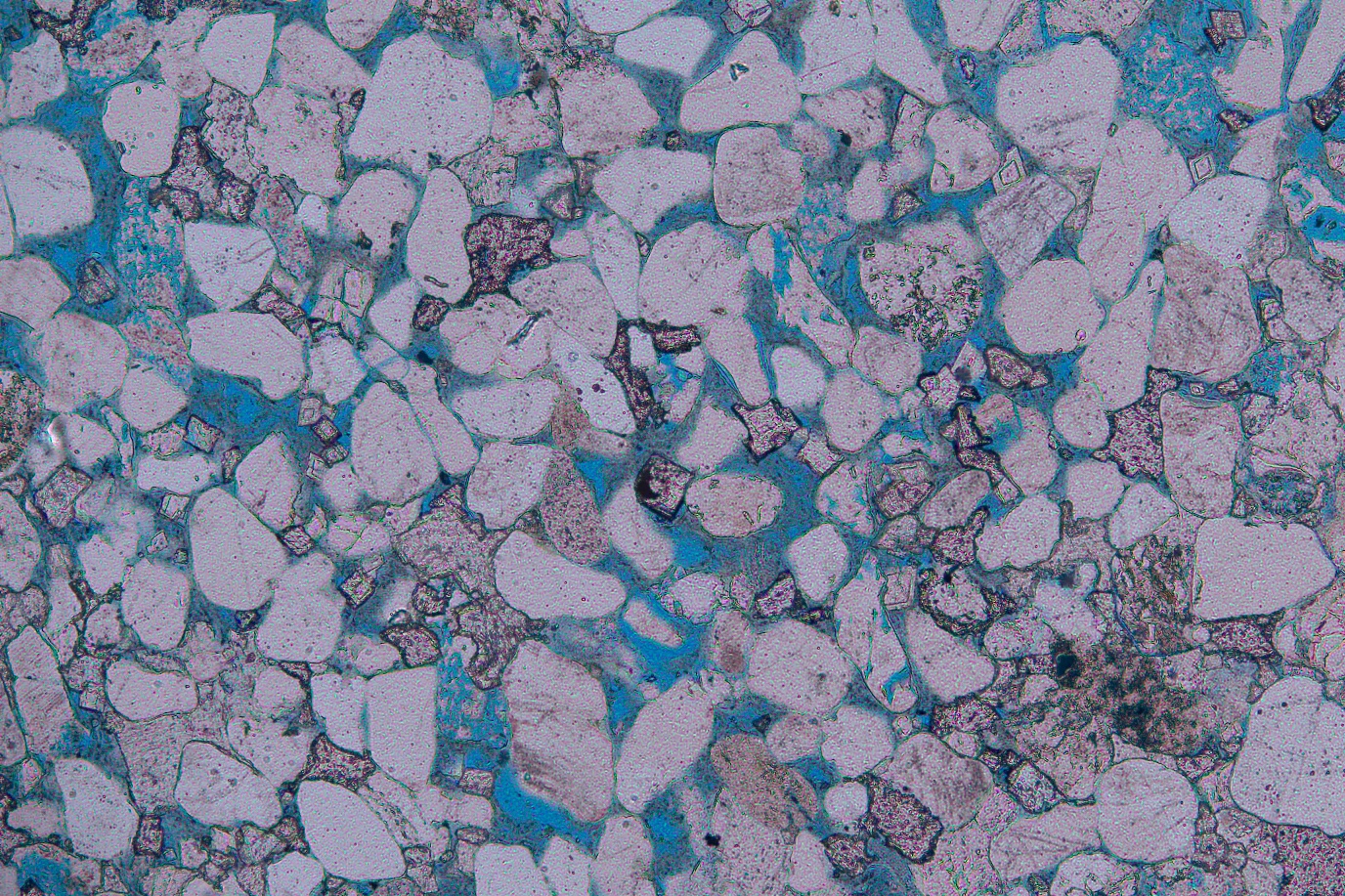
PhD students in Miocic’s group take samples from both caprock and the underlying rock material. They subject these samples to various tests in the lab. One example is nanoindentation. As Miocic explains, ‘A tiny needle pricks against the rock and measures how much force is needed before it makes an indentation. The way the needle bounces back also tells us something about the elasticity of the material.’ They repeat this measurement with a piece of rock after exposing it to hydrogen for sixty days.
Miocic’s group also uses an electron microscope to study the composition of his samples. Depending on the mineral composition, they can make predictions concerning how the rocks will be compressed by the three kilometres of layers that are pressing down on it.
See also: How to use the rocks beneath Groningen
Hydrogen will not be stored in the Groningen gas field. That’s much too sensitive politically.
The ground below Groningen does provide Miocic with his research materials, given its easy accessibility due to the gas-extraction facilities. Because the entire area of the Netherlands, including under the sea, has a similar subsurface composition, however, hydrogen could conceivably be stored somewhere in an empty offshore gas field.
Miocic focuses on two main questions: how subsurface rocks will hold up in various circumstances and how much leakage is to be expected. To this end, Miocic will take natural samples — rock fragments from below Groningen — and subject them to tests in the lab. More specifically, he will measure both the composition and strength of the rock, as well as how these characteristics might be affected by the presence of hydrogen. By entering his findings from the lab into computer models, he will be able to make predictions about subsidence and other relevant aspects.





How steel embrittles
Imagine a metal spoon. With hydrogen around, the spoon suddenly behaves like a precious piece of porcelain that breaks into pieces when it falls and hits the ground.
The research subjects of the materials scientists Bart Kooi and Francesco Maresca are not provided by the ground in Groningen, but by the Gasunie. Kooi and Maresca’s goal is to find out how the steel pipes that are currently used for natural gas would hold up if hydrogen were to be pumped through them. Leakage is one potential problem, but Kooi and Maresca are focusing their investigation on a different phenomenon: embrittlement.
Investigating theories on embrittlement
Bart Kooi explains, ‘All materials have small imperfections.’ Francesco Maresca adds, ‘Just like people. It’s the defects that makes them interesting.’ According to one theory on embrittlement, hydrogen causes beginning cracks to burst. To investigate this theory, Kooi and Maresca are collaborating with researchers from TU Delft, who will make tiny cracks in steel and then subject the material to fatigue tests, both with and without hydrogen.
These steel samples will then come to Groningen, where Kooi will be able to study their finest details with a highly advanced microscope. In this way, he will make visible how hydrogen atoms penetrate the steel. ‘When you see in the lab that the material breaks more easily in the presence of hydrogen, you’re none the wiser. We want to investigate how it breaks.’
Maresca is developing computer models at the atomic level, which also visualize the interactions between atoms in a material. Recently, one of his PhD students published an article presenting an initial model of iron. Maresca notes, ‘This is the basis. Now we will start adding carbon to obtain a model of steel. That will probably take us another year of work.’
With these models, Maresca will be able to investigate how hydrogen influences a material like steel. According to a different theory, which he will also test, hydrogen reacts with a component of steel, thus forming small regions with a different composition. This makes the material weaker at those spots.
Maresca explains, ‘We hope to learn what happens in the interaction between hydrogen and steel at the atomic level, because that is ultimately where the process of embrittlement starts.’ A better understanding of this process will eventually allow larger-scale predictions concerning how steel pipes will hold up in the presence of hydrogen.
It has been known for about 150 years that metal breaks much more easily in the presence of hydrogen. Maresca explains, ‘Imagine a metal spoon. With hydrogen around, the spoon suddenly behaves like a precious piece of porcelain that breaks into pieces when it falls and hits the ground.’
‘Hydrogen will move through pipelines at variable pressure. This varying pressure in particular will strain the material,’ notes Kooi. In that case, even a tiny crack can be fatal. The exact reason that hydrogen makes materials brittle is not known, but various theories exist. Maresca and Kooi will put several of these theories to the test in their work
The Hydrogen Valley Campus Europe is located in the Northern Netherlands. It is the place where green energy comes ashore (from wind turbines on the North Sea), where years of working with natural gas has led to a wealth of experience, and where universities and vocational institutions will work on new research and the education of the next generation of engineers for the hydrogen economy of the future. This is episode 3 of a series on hydrogen research at the Faculty of Science and Engineering at the University of Groningen.
Read more:
Hydrogen is an indirect greenhouse gas: by reacting with other compounds in the atmosphere, it may contribute to global warming in several ways.
Professor of Energy Conversion Aravind Purushothaman Vellayani is working on systems that use hydrogen to produce electricity – for large factories, for instance. But even your car or your toilet could be capable of producing electricity from hydrogen.
A sustainable way to produce hydrogen has been around for a century, but it is still much cheaper to make hydrogen from natural gas. Researchers from the UG are working on more efficient, affordable, and scalable production of green hydrogen.
Green hydrogen holds many promises. But grey hydrogen from natural gas is still much cheaper, storage of hydrogen is not trivial and as an indirect greenhouse gas is not as clean as it might look.
More news
-
17 February 2026
The long search for new physics
-
10 February 2026
Why only a small number of planets are suitable for life
